
The Phase-3 trial of Apreo Health’s bronchoscopic airway scaffold for severe emphysema and hyperinflation is now enrolling at the Temple Lung Center.
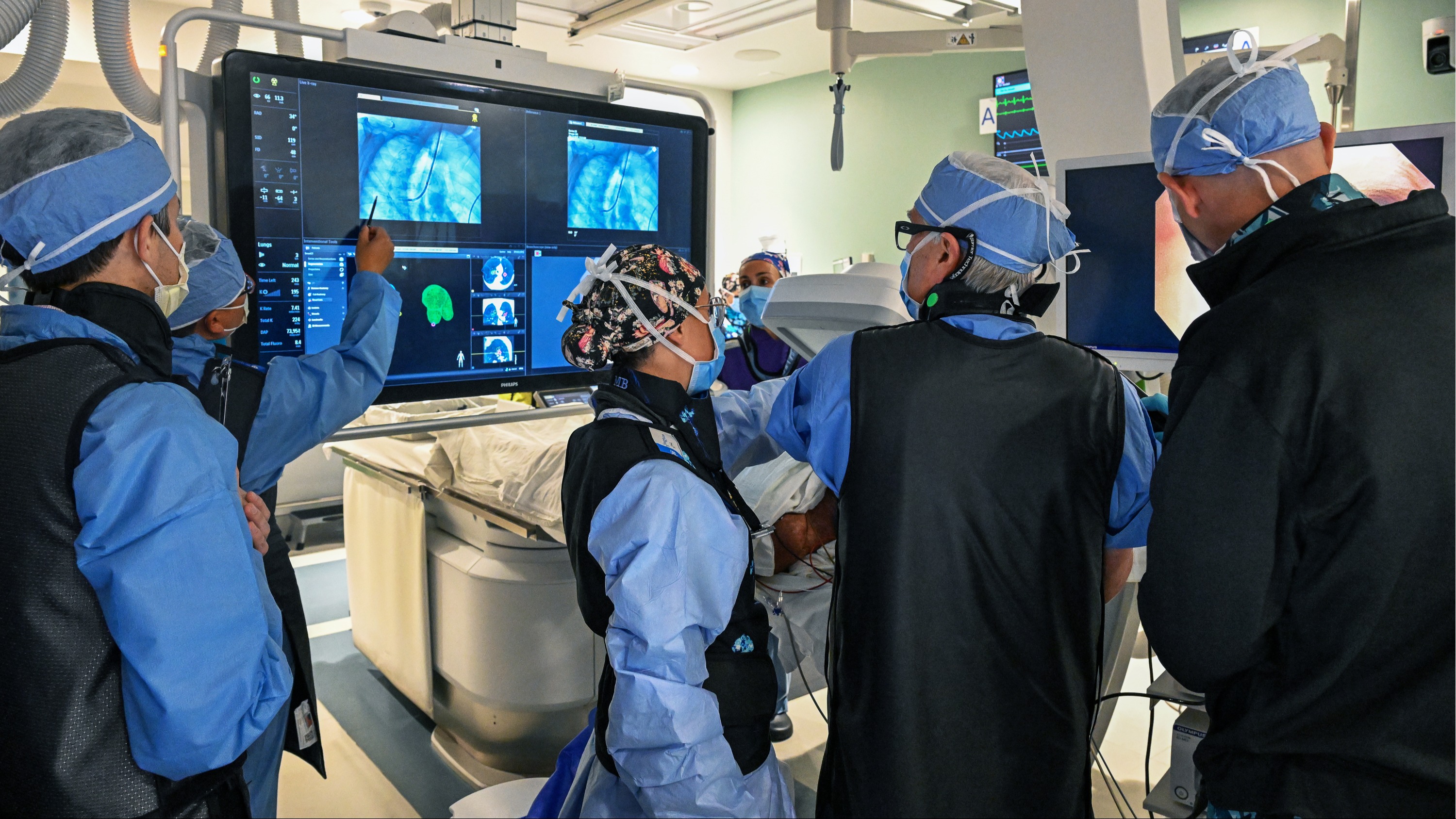
The Temple Lung Center has treated the first patient in the U.S. in the BREATHE-3 clinical trial, which will evaluate a novel bronchoscopic device designed to open airways and release trapped air in patients with severe emphysema and hyperinflation.
The procedure was led by global Primary Investigator Gerard J. Criner, MD, FACP, FACCP, Chair of Thoracic Medicine & Surgery at the Lewis Katz School of Medicine and Director of the Temple Lung Center, continuing Temple’s 35+ years of leadership in interventional therapies for COPD/emphysema.
The Impact for Patients with COPD
Emphysema is a type of chronic obstructive pulmonary disease (COPD) that makes it hard to breathe. It is progressive and worsens over time. The condition affects the air sacs in the lungs, making it harder for the lungs to move oxygen in and carbon dioxide out of the body.
The BREATHE-3 clinical trial will enroll up to 250 participants across 25 centers in the U.S. and Europe and will follow them for three years. Those between the ages of 40-84 with severe COPD-related emphysema and significant breathlessness may be eligible to participate.
Refer your patients today.
The Temple Lung Center is home to one of the most robust research hubs for pulmonary disease in the nation, and offers one of the most comprehensive COPD programs in the world, delivering internationally-recognized expertise in pioneering breakthrough interventions and advanced treatment options. The multidisciplinary team of physician-researchers collaborates closely with referring physicians, ensuring collaborative approaches to personalized patient care.
Our team will screen for eligibility, review alternatives (including other minimally invasive options, access to alternative clinical trials, or surgical options when necessary), and communicate findings back to your team.
Eligibility Criteria
Patients may be eligible to join these studies if they meet the criteria below (list is not exhaustive):
- Subject is at least 40, but not older than 84, years of age.
- Subject has body mass index (BMI) of between 18 and 32
- Subject has completed a documented pulmonary rehabilitation program within 12 months prior to Baseline.
For more information about this or other clinical research trials at the Temple Lung Center, email breathebetter@tuhs.temple.edu or call 215-707-1359.

